| By: Paul S. Cilwa | Viewed: 4/19/2024 Posted: 1/6/2010 |
Page Views: 5267 | |
| Topics: #Geology #Coal #Out-of-PlaceArtifacts | |||
| Let's look at coal: Its formation, mining, uses, legends, and what odd artifacts have been found embedded in it. | |||
When my kids were young and asked me what I was getting them for Christmas, I always replied, "A bag of coal." Of course, I never gave them coal for Christmas, or at any other time. And in fact, few modern Americans have even seen coal, though they think they have. That's because most people think charcoal is the same as coal; and everyone has seen charcoal briquettes at barbeques, right?
But charcoal is not coal. And coal is way more interesting. (For starters, kids getting coal for Christmas was once considered a good thing!)
First of all, the basic difference: coal is a mineral that comes from the ground. Unlike most minerals, it burns. It's been known about since ancient times.

Charcoal is wood or any flammable substance that has been heated and dried enough to reduce it to almost pure carbon, with just a few impurities. It resembles coal, both in appearance and in the fact that it can be burnt. In fact, it's name comes from Old English charren coal which means "turn into coal". Our modern word "char" (meaning "scorch") is a back-formation from charcoal.
The purpose of creating charcoal then, as now, is to make transport of wood for fires easier. Wood is filled with water; and the water component of wood doesn't burn. So transporting light charcoal is much more efficient than transporting wood.
Charcoal, then, is fake coal. So, what is real coal?
Coal was first described in 370 BCE (almost 2500 years ago) by Theophrastus in his treatise "On Stones" under the name lithos anthrakos (English: anthrax; the stone, not the disease). More recently, in Scotland and in New England a gift of coal on New Year's Day was a token of good luck, guaranteeing a "warm hearth" for the coming year.
Although coal was recognized by the ancient Greeks, it wasn't in great demand for 2,000 years. After all, wood was plentiful and renewable and a lot easier to get to than digging underground for coal. Thus, when a Greek wanted to say that it was silly to bring something to a place where there was already lots of that stuff, he would say it was like "bringing owls to Athens." The phrase "bringing coals to Newcastle" was first recorded in 1538 well after Newcastle had become world-famous as a source of coal.
Coal doesn't always have to be dug. Like any mineral, coal is sometimes found in outcroppings where erosion has exposed deeper layers of earth to the air. Because coal burns hotter than wood, it is useful in funeral pyres and its use has been detected in pyres in Bronze Age Britain (4000-5000 years ago). By 300 CE the Romans were exploiting coal fields in England and Wales.
But by 1000 CE the easily accessible coal supplies had been exhausted, and coal had enough specialized uses to justify mining.
There was originally a resistance to mining of any sort. Many cultures considered it to be a "raping" of Mother Earth. However, as big money was to be had in coal, local religions were persuaded (via large donations) to "explain" to the congregations that "Mother Earth" did not exist, and Father God had given these things to humanity for us to do with as we pleased. Anyone who disagreed was "raked over the coals" as a heretic, which has always been a particularly effective means of squelching dissent. (Note: The coals were burning at the time.)
It was the development of a practical steam engine that suddenly propelled coal into the limelight as a premier energy source.
The earliest known steam engine was the aeolipile described by Hero of Alexandria. But neither it, nor the experimental devices that followed in places as diverse as Turkey (1551) and Italy (1621) were practical enough to do real work. That changed in 1712 when Thomas Newcomen introduced his "atmospheric engine", and shortly afterwards when James Watt's redesign of Newcomen's engine used 75% less coal to do the same work. So significant was Watt's contribution that today, we use Watt's name (the watt) as a measurement of the work a given amount of energy can achieve.
Watt's steam engine made it possible to build a factory that did not rely on a water wheel and therefore did not need to be located on the banks of a stream. Thus was born the Industrial Revolution.
One might ask, why would people leave an idyllic life on the family farm and move to the city to work in a factory? Of course, today most people don't live in the country and so can't imagine anything more dreary than farm life. But remember, people love what they're used to. And British of the 1700s did not want to leave their farms.
So Parliament, which was composed mostly of industrialists, passed laws that heavily taxed farms. Unable to pay their taxes, farmers were forced to leave their homes and went to the city to find work…which was conveniently to be had in the factories the industrialists owned. However, factories paid notoriously low wages. For a family to survive, everyone, even very young children, had to work. That, of course, meant no school. The family and its descendents were mere fodder for the machines.
Bertrand Russell described conditions thus:
The industrial revolution caused unspeakable misery both on England and in America. … In the Lancashire cotton mills (from which Marx and Engels derived their livelihood), children worked from 12 to 16 hours a day; they often began working at the age of six or seven. Children had to be beaten to keep them from falling asleep while at work; in spite of this, many failed to keep awake and were mutilated or killed. Parents had to submit to the infliction of these atrocities upon their children, because they themselves were in a desperate plight. Craftsmen had been thrown out of work by the machines; rural labourers were compelled to migrate to the towns by the Enclosure Acts, which used Parliament to make landowners richer by making peasants destitute; trade unions were illegal until 1824; the government employed agents provocateurs to try to get revolutionary sentiments out of wage-earners, who were then deported or hanged. Such was the first effect of machinery in England.
It was an industrialist's wet dream, until by 1847 laws passed (pushed hard by liberals of the time, and fought by conservatives) limiting workers to a mere 10 hours per day.
By the way, while child labor is no longer legal in America or Europe, it still is very active in many parts of the world. Pretty much anything you buy from Wal-Mart was manufactured by Chinese four-year-olds between their cigarette breaks.

And coal, China's primary source of electrical power (which is made by turning electrical generators by steam power), is still behind it all…an unexpected fate for a mineral that was mostly formed between 360 and 299 million years ago.
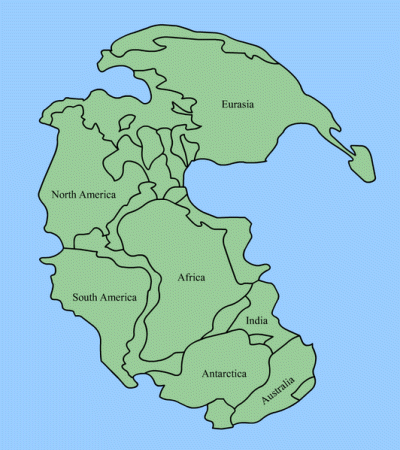
At that time, which we now call the "Carboniferous Period," all the tectonic plates that make up Earth's land masses had floated together to make up a single supercontinent we call Pangaea. (The southern regions are sometimes referred to as Gondwana.) That means weather was markedly different than ours. At the start of the Carboniferous Period, earth's atmosphere was heavily laden with carbon dioxide which, as we all now know, is a "greenhouse gas" that holds in the Sun's heat. So at the beginning of this period earth's average temperature was relatively high. Most of Pangaea was stretched out around the equator, so on it developed something that had never existed before on Earth: a forest.
This wasn't just any forest. Remember, all the land masses of Earth were squashed together and most mountains had not yet formed. So this forest was low, swampy, and unbroken: Think the Okefenokee Swamp times 135,000, except with 200-foot-tall trees with six-foot-thick trunks. Oh, and the "trees" were actually giant ferns.

Still, a swamp is a swamp, and that means a lot of shallow, oxygen-poor water. When these giant trees fell, they fell into the water; and because there wasn't much oxygen there wasn't a lot of bacteria—and, therefore, the trees didn't rot; they just laid there, where more trees fell on top of them and still more trees fell on top of them. The weight squashed them flat, and still more trees fell…for 60 million years, almost as long as the time between the dinosaurs and the present day.
Now, as I said, at the beginning of the Carboniferous Period there was a lot of carbon dioxide in the atmosphere. But as these trees grew, they absorbed the carbon dioxide and exhaled oxygen, as all plants do. However, because of the swamp, fallen trees did not rot and therefore didn't release that carbon dioxide when they died. They simply kept it. And gradually, over those 60 million years, the atmosphere lost much of its carbon dioxide and gained a lot of oxygen, much more than we have today (which had the very cool effect of allowing the growth of giant insects, such as six-foot centipedes and dragonflies with two-foot wingspans).

The Carboniferous Period came to end when the loss of greenhouse gas resulting in an abrupt Global Cooling. The southern portion of Pangaea became glaciated as an ice age began, which carried into the Permian Period that followed.
With the passing of more millions of years and the continued movement of the tectonic plates, which go up and down as well as back and forth, the layers that had been tree-filled swamp subsided and oceans came and went above, depositing layers of limestone on top. Eventually as much as 2,000 feet of rock formed above the trees, adding intense heat and pressure to the dead vegetation. This squeezed out any water and broke down the more complex molecules, leaving coal (and sometimes oil and natural gas) behind.

In the United States, only about 40% of our coal comes from deep mines (the rest is closer to the surface). Most texts tell us (or imply) that all coal was formed during or shortly after the Carboniferous Period, and that the difference in depth is due to differences in geologic deposition and erosion during the intervening 299,000,000 years.
And yet there are some so-called OOPArts (Out-of-Place Artifacts) associated with coal. These artifacts, which are admittedly very rare, nevertheless shouldn't occur at all if the story that all coal comes from the Carboniferous is correct.
For example: In 1891, Mrs. S. W. Culp, of Morrisonville, Ill. was fragmenting coal into smaller pieces for her kitchen stove when she noticed a chain stuck in the coal. The chain measured about 10 inches long and was later found to be made of eight-carat gold, and described as being "of antique and quaint workmanship." According to the Morrisonville Times of June 11, 1891, investigators concluded that the chain had not simply been accidentally dropped in with the coal, since some of the coal still clung to the chain, while the part that had separated from it still bore the impression of where the chain had been encased.
I shouldn't have to mention that there were no mammals, let alone humans, to work gold into chains 300 million years ago.

And that's not the only example. A brass bell (made of an odd combination of metals that is not typical of any historical examples of brass) was found in 1944 in a coal deposit in West Virginia. The investigators who studied the bell couldn't disprove the finding but they couldn't explain it, either.
What's obvious to me is that coal doesn't have to cook for 300 million years. A few thousand, in fact, might be sufficient if conditions are right.
For example, suppose that some 13,000 years ago a meteorite smashed into what is now Hudson Bay, causing a mega-tsunami to encircle the Earth. It's not too hard to imagine some kind of civilization in existence then that would have been wiped out. The forests of the world would have been destroyed at the same time, flattened and swept out to sea and gathering in lake bottoms. All this dead matter would have caused a "bloom" of bacteria that would quickly suck the oxygen out of the lakes and seas and the vegetable matter would have also been covered by loose gravel, sand, rocks, and anything else the tsunami could pick up, providing the required pressure. If the debris washed along with the trees included the occasional bell or gold chain, well, they'd have become inextricably connected to the coal as it formed.
Is that what happened? Well, I certainly can't prove it. All I know is, there are OOPArts found in coal, as well as in other rocks and gems where they shouldn't be possible. Too bad that carbon dating wasn't invented back in 1891.
A carbon-dated lump of coal with a gold chain embedded in it: That's what I want in my stocking for Christmas!





